Northern Meditec HF3 has Obtained EU Authoritative Certification! Innovative Technology Leads Respiratory Therapy to a New Level.
Author:
none
Origin:
none
Time of issue:
Views:
(Summary description):
Northern Meditec HF3 (High-Flow Nasal Cannula Respiratory Humidifiers) Successfully Obtained MDR Certification and Started a New Journey in the International Market.

The HF3 (High-Flow Nasal Cannula Respiratory Humidifiers) independently developed by Northern Meditec recently obtained the EU Medical Device Regulation (MDR) certification. This milestone breakthrough not only indicates that Northern Meditec’s product quality, safety and performance have reached the strict standards of the European market, but also lays a solid foundation for further expansion of the international market.
On 30th April 2025, the High-Flow Nasal Cannula Respiratory Humidifiers (HF3) of Northern Meditec, officially passed the strict review of the SGS FIMKO OY Notified Body 0598 and obtained the quality management system certificate in compliance with the EU Medical Device Regulation (EU) 2017/745 Annex IX. This certification covers risk class IIa medical devices, indicating that the entire process of the product from research and development, production to clinical application has met the strict EU standards.
EU MDR Certification
The “Gold Standard” for Global Medical Device Regulation
The MDR certification is a certification system under the new EU medical device regulations. The certification regulations and standards are known for their strict clinical data review and full life cycle quality management. The certification requires multi-dimensional evaluations such as biocompatibility, mechanical properties, and sterilization verification. For medical device companies, obtaining MDR certification is not only a huge challenge, but also shows that our HF3 (High-Flow Nasal Cannula Respiratory Humidifiers) has met the highest EU regulatory requirements in terms of safety, reliability, and long-term stability.
The HF3 (High-Flow Nasal Cannula Respiratory Humidifiers) has passed the MDR certification, which means that it can be freely circulated in the EU and related countries, and deeply participate in the global high-end medical market competition. This is not only an important milestone in Northern Meditec’s internationalization journey but also demonstrates the leapfrog progress of “China Smart Manufacture” from a single product breakthrough to the export of the entire product line.

Technology Accumulation for 13 Years
Enabling Breakthroughs in International Certification
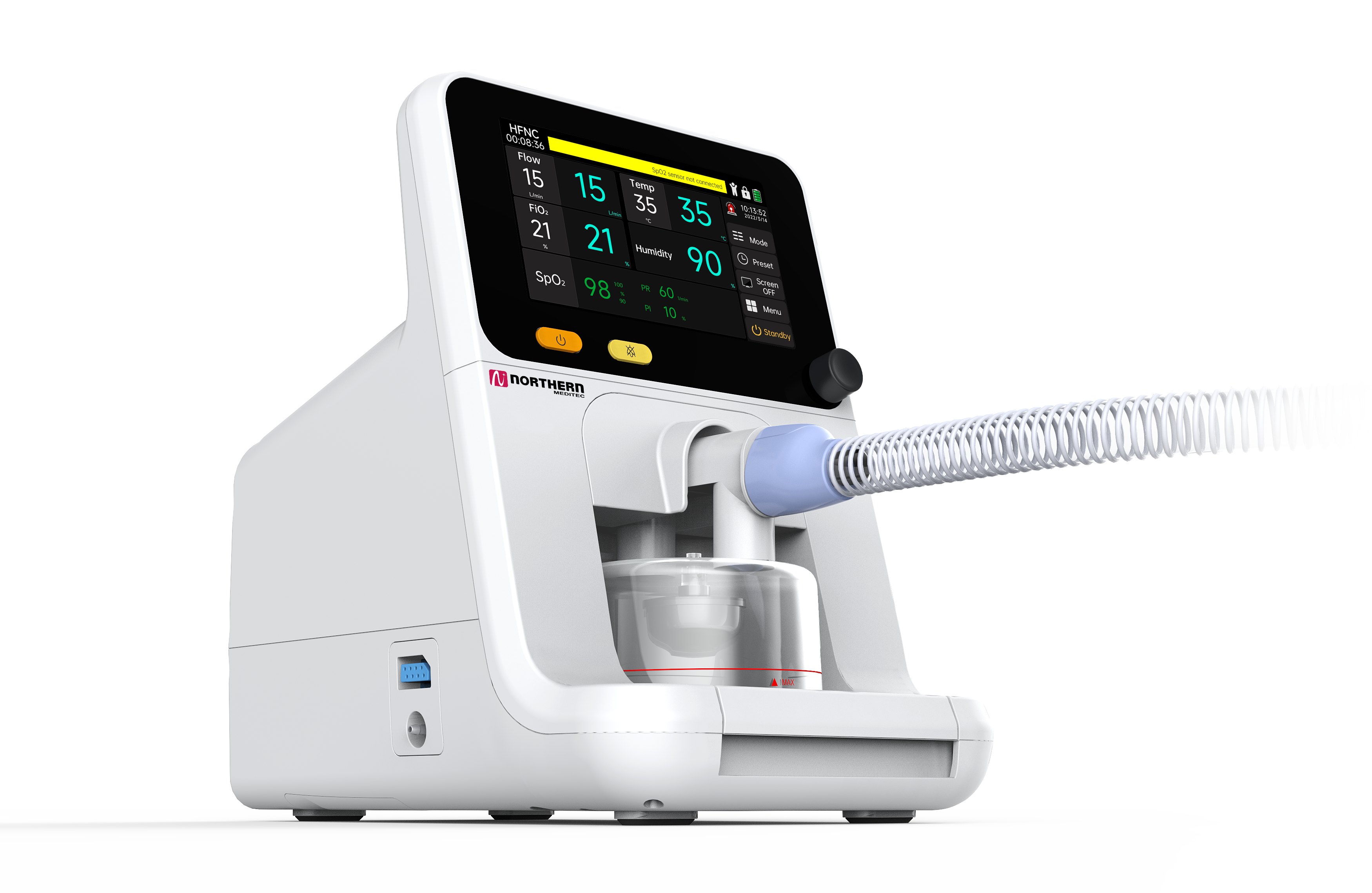
Northern Meditec’s HF3 can continuously provide patients with adjustable and relatively constant Oxygen Concentration (21%-100%), Temperature (31-37°C) and Flow (8-80L/min) of inhaled gas through high-flow dedicated nasal plugs or other patient interfaces. The device is commonly used in patients with mild to moderate hypoxemia, such as patients with hypoxic respiratory failure, ARDS, pneumonia, pulmonary fibrosis, cardiogenic pulmonary edema and other diseases. It has a positive therapeutic effect on patients with simple hypoxic respiratory failure (Type I Respiratory Failure) and also shows certain potential in the treatment of some patients with mild hypoxia combined with hypercapnia (Type II Respiratory Failure).
Technology Going Global
Starting a New Journey of Globalization



Related news








